37++ General Formula Of Alkane Alkene Alkyne
General Formula Of Alkane Alkene Alkyne. This can be verified from the above diagram as well. Alkene:—aliphatic unsaturated hydrocarbons in which carbon atoms are attached with each other by means of double bonds are called as alkene.

[c_{n}h_{2n+2}] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two. The general equation for the burning or combustion of an alkane, an alkane or an alkyne with x number of carbon atoms (cx) and y number of hydrogen atoms (hy) is: Alkynes are the unsaturated hydrocarbons that have at least one carbon and carbon triple bond.
in wall toilet brush holder grillage footing housse coussin bleu marine idealism meaning
Alkane Alkene Alkyne Worksheet Imperialdesignstudio
The general formula for alkene is c n h 2 n , where n is any integer greater than 1.the simplest alkene is ethene containing two carbons and having molecular formula c 2 h 4. Alkanes have a general formula: General formula for alkenes is cnh2n. Their general molecular formula is ,cnh2n.

Alkanes have the general formula cnh2n+2 and can be subdivided into the following three groups: [c_{n}h_{2n+2}] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two. Their general molecular formula, cnh2n+2. The suffix ‘ane’ is generally used to describe alkanes. The straight chain alkanes share the same general.

What is the general molecular formula for alkenes. C x n h 2 n + 2. Which formula is the general formula for an alkane? The most common alkyne is ethyne, better known as acetylene. Examples for the nomenclature of alkanes as per iupac guidelines include methane for the compound ch 4 and butane for

Alkanes have the general chemical formula c n h 2n+2. The general equation for the burning or combustion of an alkane, an alkane or an alkyne with x number of carbon atoms (cx) and y number of hydrogen atoms (hy) is: Methane gas is the first member of the homologous series of alkanes. The straight chain alkanes share the same.
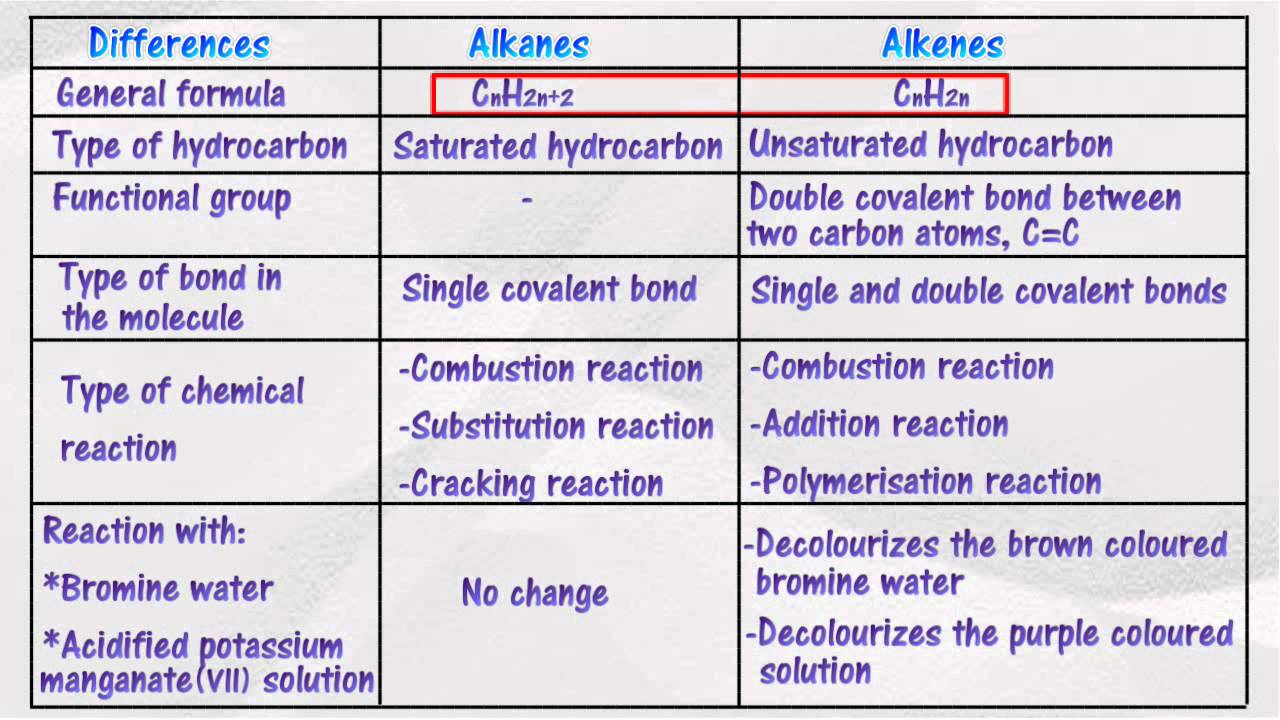
You can use these generic formulas to predict the formula for any alkane, alkene, or alkyne. Their general molecular formula is ,cnh2n. The longest continuous chain should include both the carbon atoms of the triple bond. Alkenes have the general formula c n h 2n. If the carbon number is 5 i.e.

Alkenes have at least one double bond and alkynes have at least one triple bond. This can be verified from the above diagram as well. Methane gas is the first member of the homologous series of alkanes. Alkenes and alkynes are unsaturated hydrocarbons. General formula for alkenes is cnh2n.

Their general molecular formula is ,cnh2n. If the carbon number is 5 i.e. General formula for alkenes is cnh2n. C x n h 2 n + 2. The suffix ‘ane’ is generally used to describe alkanes.

As with other organic compounds, the carbon atoms in alkanes may form straight chains, branched. Where n= number of carbon atoms. [c_{n}h_{2n+2}] the general formula means that the number of hydrogen atoms in an alkane is double the number of carbon atoms, plus two. Click in the answer box to activate the palette. What is the general molecular formula for.

Their general formula is cnh 2n. Alkenes and alkynes are unsaturated hydrocarbons. Pentane, we shall have c 5 h 2(5) + 2 meaning c 5 h 12. The general formula for alkanes is cnh2n+2, where n stands for number of carbon atoms and 2n+2 for number of hydrogen atoms in the molecule. Alkenes have the general formula c n h.