18++ Hydrochloric Acid And Sodium Hydroxide Ionic Equation
Hydrochloric Acid And Sodium Hydroxide Ionic Equation. Nitric acid + calcium hydroxide → 2. Sodium hydroxide + hydrochloric acid = sodium chloride + water naoh + hcl = nacl + h2o

See the answer see the answer done loading. Nitric acid + calcium hydroxide → 2. The reaction of perchloric acid and sodium hydroxide represents a net ionic equation involving a strong acid and strong base.
hauteur wc handicap geberit imagenes png stickers hour of code 2018 promo video idee coiffure soiree cheveux mi long
Hydrogen Gas Barium Reacts With Hydrogen Gas
Babr 2 (aq) + na 2 so 4 (aq) baso 4 (s) + 2 nabr(aq) ionic equation: Sodium hydroxide (naoh) is a base, whereas hydrochlolic acid (hcl) is an acid. Net ionic equation for the. 1) hydrochloric acid and sodium hydroxide complete ionic:

Hcl (aq) + nh 3(aq) nh 4cl (aq) net ionic equation: Sodium hydroxide hydrochloric acid balanced molecular equation hydrochloric acid and sodium hydroxide equation, heat of reaction between hydrochloric acid and sodium hydroxide water h2o the reaction equation is naoh hcl nacl h2o in words sodium hydroxide plus hydrochloric acid gives sodium chloride and water. In order to write the.

Babr 2 (aq) + na 2 so 4 (aq) baso 4 (s) + 2 nabr(aq) ionic equation: Write the net ionic equation for: Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (the salt) and water. For the net ionic equation, we would have. For example, consider the reaction described by the following full molecular equation:
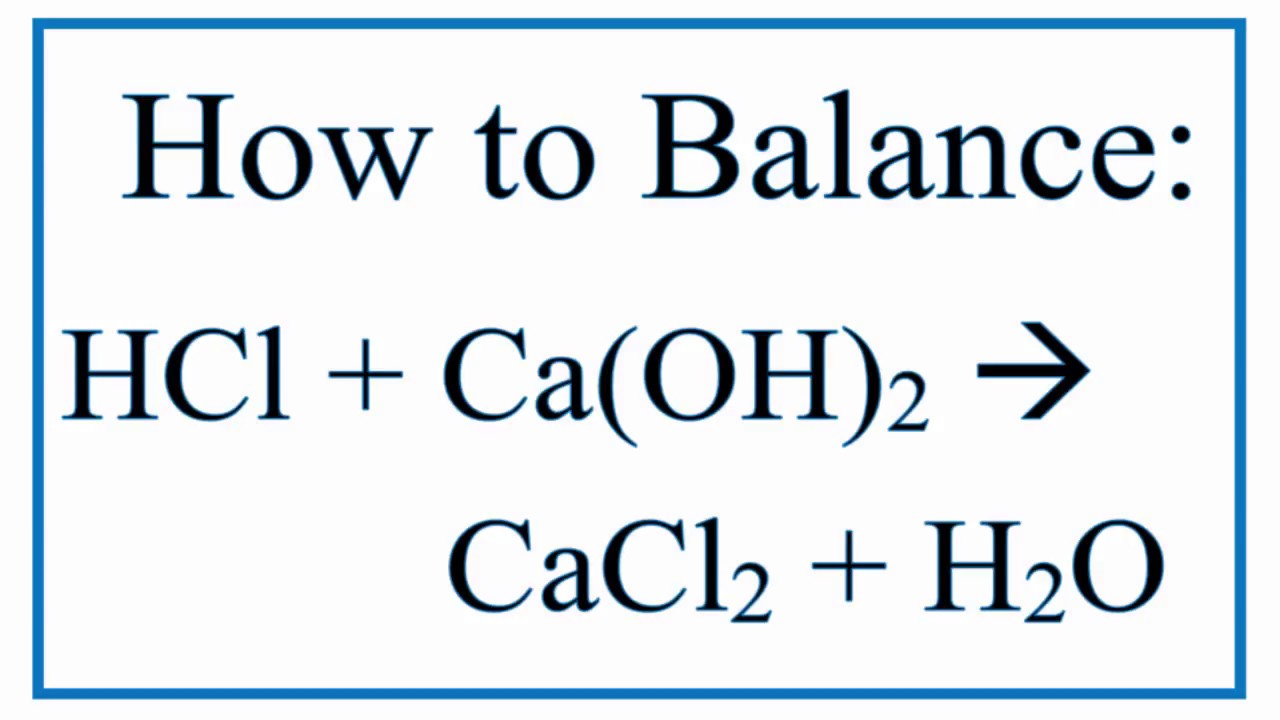
Hydrofluoric acid + potassium hydroxide → chemistry. Naoh (aq) + hcl (aq) = > nacl (aq) + h2o (l) nacl is the salt also known as common salt, h2o is water. The reaction of acetic acid with sodium hydroxide. Sodium hydroxide (aq) + hydrochloric acid (aq) rightarrow observation: Naoh(aq) → na+ (aq) +oh− (aq) hcl(aq) → h+ (aq) +cl− (aq).

See the answer see the answer done loading. Nitric acid + calcium hydroxide → 2. There are three main steps for writing the net ionic equation for hcl + koh = kcl + h2o (hydrochloric acid + potassium hydroxide). Write the net ionic equation for: Strong acids and strong bases are considered strong electrolytes and will dissociate completely.

Barium chloride (aq) + sodium carbonate (aq) rightarrow This chemistry video tutorial explains how to write the net ionic equation between sodium hydroxide and hydrochloric acid. Finally, what is the ionic equation for hydrochloric acid and sodium hydroxide?, let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric.

Hcl (aq) + nh 3(aq) nh 4cl (aq) net ionic equation: There are three main steps for writing the net ionic equation for hcl + koh = kcl + h2o (hydrochloric acid + potassium hydroxide). Naoh(aq) → na+ (aq) +oh− (aq) hcl(aq) → h+ (aq) +cl− (aq) now, when these two solutions are mixed, the hydroxide anions produced by the.

Sodium hydroxide hydrochloric acid balanced molecular equation hydrochloric acid and sodium hydroxide equation, heat of reaction between hydrochloric acid and sodium hydroxide water h2o the reaction equation is naoh hcl nacl h2o in words sodium hydroxide plus hydrochloric acid gives sodium chloride and water. A handy rule of thumb for acid base reactions master. Hcl(aq) + naoh(aq) nacl(aq) + h2ohcl,.

See the answer see the answer done loading. The reaction of perchloric acid and sodium hydroxide represents a net ionic equation involving a strong acid and strong base. Hydrochloric acid + sodium carbonate → sodium chloride + water + carbon dioxide gas 4. This problem has been solved! Hclo 4 + naoh = naclo 4 + h 2 o is.