13++ Hydrochloric Acid And Sodium Hydroxide Reaction Type
Hydrochloric Acid And Sodium Hydroxide Reaction Type. Sodium carbonate and hydrochloric acid reaction type sodium carbonate and hydrochloric acid reaction type. Which substance is always produced in the reaction between hydrochloric acid and.
As the reacting acid is hydrochloric acid, then the salt produced will be a chloride. 1 m calcium chloride solution. Is the reaction between sodium hydroxide and hydrochloric acid endothermic or exothermic?
geberit toilettenspulung reparieren horloge legrand pour ballon deau chaude grille de ventilation acoustique pour fenetre housse de coussin 40 x 60 cm
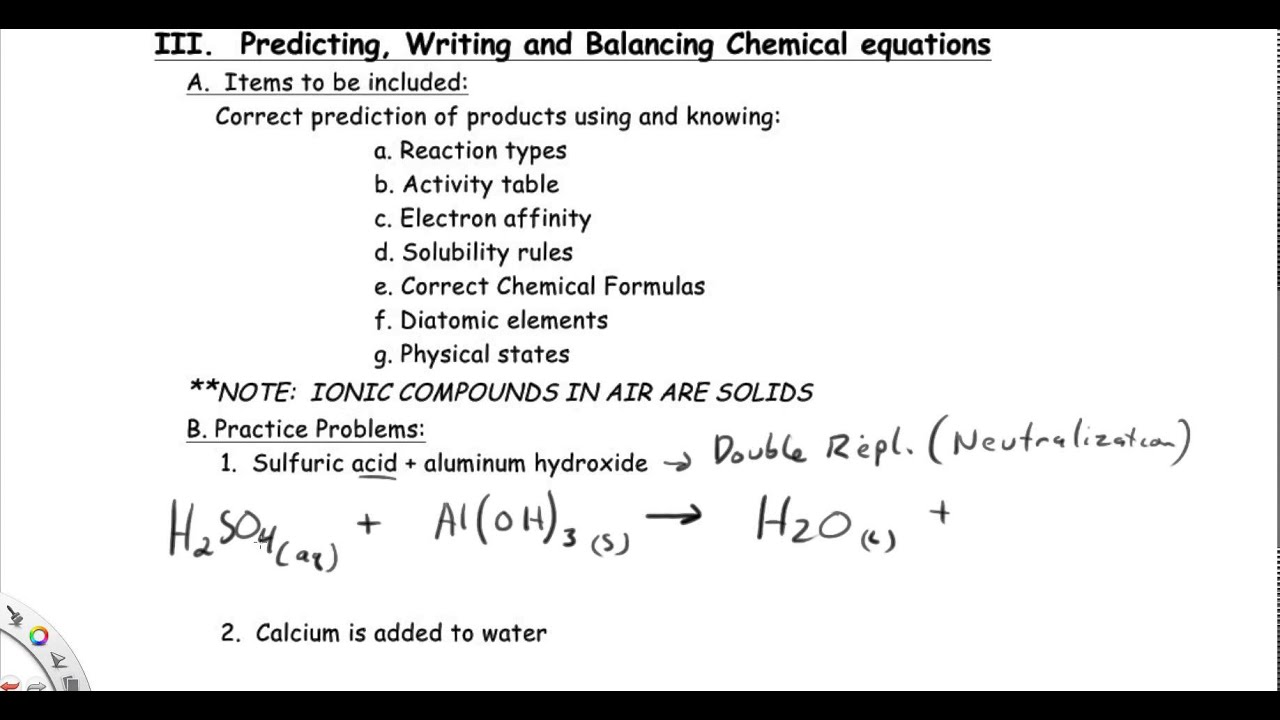
Sulfuric acid plus aluminum hydroxide YouTube
1 m calcium chloride solution. Hydrochloric acid + aqueous sodium hydroxide 7. Preparation and standardization of 0.1 m sodium. Hydrochloric acid and the base sodium hydroxide react to form the salt sodium chloride and water.

2 days agosodium carbonate and hydrochloric acid reaction type. Sodium chloride is a salt. Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (the salt) and water. Which substance is always produced in the reaction between hydrochloric acid and. Odourless white crystals (nacl) is observed.

Since there is no overall transfer of electrons, this can't be a redox reaction, and that means activities won't matter here. The reaction between sodium hydroxide (naoh) and hydrochloric acid (hcl) is a neutralization reaction which results in the formation of a salt, sodium chloride (nacl) , and water (h2o). It is an exothermic reaction. This double replacement makes this.

Despite the aggression of the sodium hydroxide and hydrochloric acid, the reaction was a wonderful one. Is hydrochloric acid stronger than sodium hydroxide? Hydrochloric acid and sodium hydroxide reaction type. Is the reaction between sodium hydroxide and hydrochloric acid endothermic or exothermic? This double replacement makes this a double replacement reaction.

Now as per the law of conservation of mass, mass is neither created nor destroyed in a chemical reaction. Despite the aggression of the sodium hydroxide and hydrochloric acid, the reaction was a wonderful one. Which substance is always produced in the reaction between hydrochloric acid and. Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen.

Sodium carbonate and hydrochloric acid reaction type sodium carbonate and hydrochloric acid reaction type. Solved the reaction between sodium hydroxide and | chegg.com. Is the reaction between sodium hydroxide and hydrochloric acid endothermic or exothermic? Sodium carbonate and hydrochloric acid reaction type The big idea for most calorimetry themed demonstrations is energy is conserved.

Hydrochloric acid reacts with sodium hydroxide to form sodium chloride (the salt) and water. What type of reaction is displayed below hcl naoh → nacl h2o? This type of reaction is called neutralisation reaction. We investigated the best remediation strategies to maximize arsenic removal efficiency for both soi. In the reaction, sodium chloride ( nacl) is formed.

1 m calcium chloride solution. Preparation and standardization of 0.1 m sodium. 2 days agosodium carbonate and hydrochloric acid reaction type. We investigated the best remediation strategies to maximize arsenic removal efficiency for both soi. The given reaction is as follows:

Aqueous solutions of sodium hydroxide and hydrochloric acid are mixed. It is an exothermic reaction. What is the difference between hydrochloric acid and hydrogen chloride? Sodium hydroxide ionizes in water to form sodium ions and hydroxide ions; Since there is no overall transfer of electrons, this can't be a redox reaction, and that means activities won't matter here.