48++ Hydrochloric Acid Burning Metal
Hydrochloric Acid Burning Metal. When zinc metal is mixed with hydrochloric acid what is produced? Next, place a burning splint near the mouth of the test tube to test for the presence of hydrogen gas.

The gas evolved extinguishes a burning candle. It also reacts with water, releasing heat. Place approximately 1 g of the magnesium pieces in the test tube, but do not add the hydrochloric acid until everything.
image de chambre de fille de 10 ans home composting bin gouttiere zinc prix genesis 2 18 in malayalam
Hydrogen Gas Ks3 Test For Hydrogen Gas
When the soap bubbles start to float up, hold a. The gas evolved extinguishes a burning candle. The hydrogen chloride gas is derived from the burning of chlorine and hydrogen. When zinc metal is treated with a dilute solution of a strong acid, a gas is evolved, which is utilised in the hydrogenation of oil.

Magnesium metal and hydrochloric acid solution place one scoop of magnesium turnings into the test tube. It is also used in aluminum etching and metal cleaning applications. A metal compound reacts with dilute hydrochloric acid to produce effervescence. A metal compound reacts with dilute hydrochloric acid to produce effervescence. The metal aluminium dissolves in hydrochloric acid, producing aluminum chloride and.
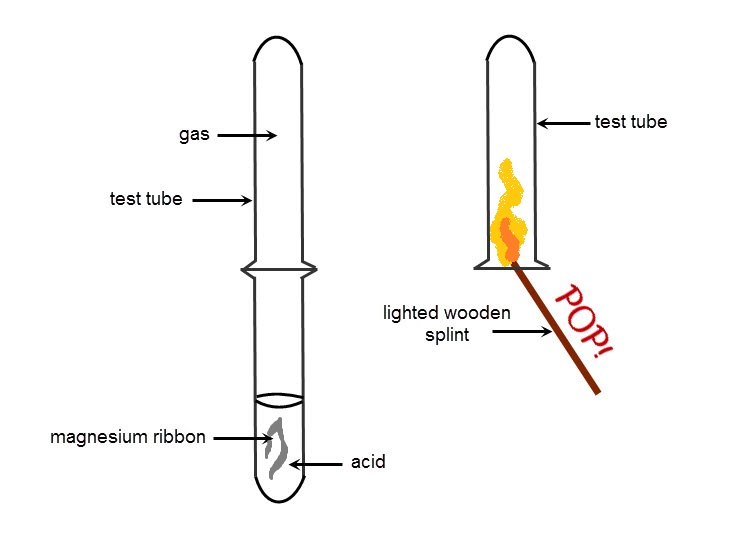
Hydrogen chloride has many uses, including cleaning, pickling, electroplating metals, tanning leather, and refining and producing a wide variety of products. Upon contact with water, it forms hydrochloric acid. When the soap bubbles start to float up, hold a. Zinc metal reacts with hydrochloric acid to. Next, place a burning splint near the mouth of the test tube to test.

The gas evolved extinguishes a burning candle. This can be proven using a burning splint because. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. Magnesium boride treated with concentrated hydrochloric acid produces spontaneously flammable gas. Place approximately 1 g of the magnesium pieces in the test tube, but do not add.

Chromium metal dissolves in dilute hydrochloric acid to form solutions containing the aquated cr(ii) ion together with hydrogen gas, h2. Metal compound a reacts with dilute hydrochloric acid to produce effervescence. Calcium plus hydrochloric acid gives us calcium chloride. Place approximately 1 g of the magnesium pieces in the test tube, but do not add the hydrochloric acid until everything..

When the magnesium reacts with the acid, the evolved hydrogen gas is collected by water displacement and its volume measured. Similar results are seen for sulphuric acid but pure samples of chromium may be resistant to attack. Hydrogen chloride is used for cleaning, pickling, and electroplating metals; A metal compound reacts with dilute hydrochloric acid to produce effervescence. Hydrochloric acid.

Place approximately 1 g of the magnesium pieces in the test tube, but do not add the hydrochloric acid until everything. Add approximately 40 ml of hydrochloric acid and immediately place the stopper on the test tube. When a metal is put in acid,. Then, what’s the name of the metal compound? Rust is a mixture of iron oxide and.